Plasma State of Matter
What is Plasma?
Plasma is the fourth state of matter, often called the "fourth state." Unlike solids, liquids, and gases, plasma consists of ionized gases where atoms have lost electrons, creating a mixture of free electrons and positive ions. Plasma is highly energetic and responsive to electromagnetic fields. It is the most abundant state of matter in the universe, making up most of the sun, stars, and the interiors of planets.
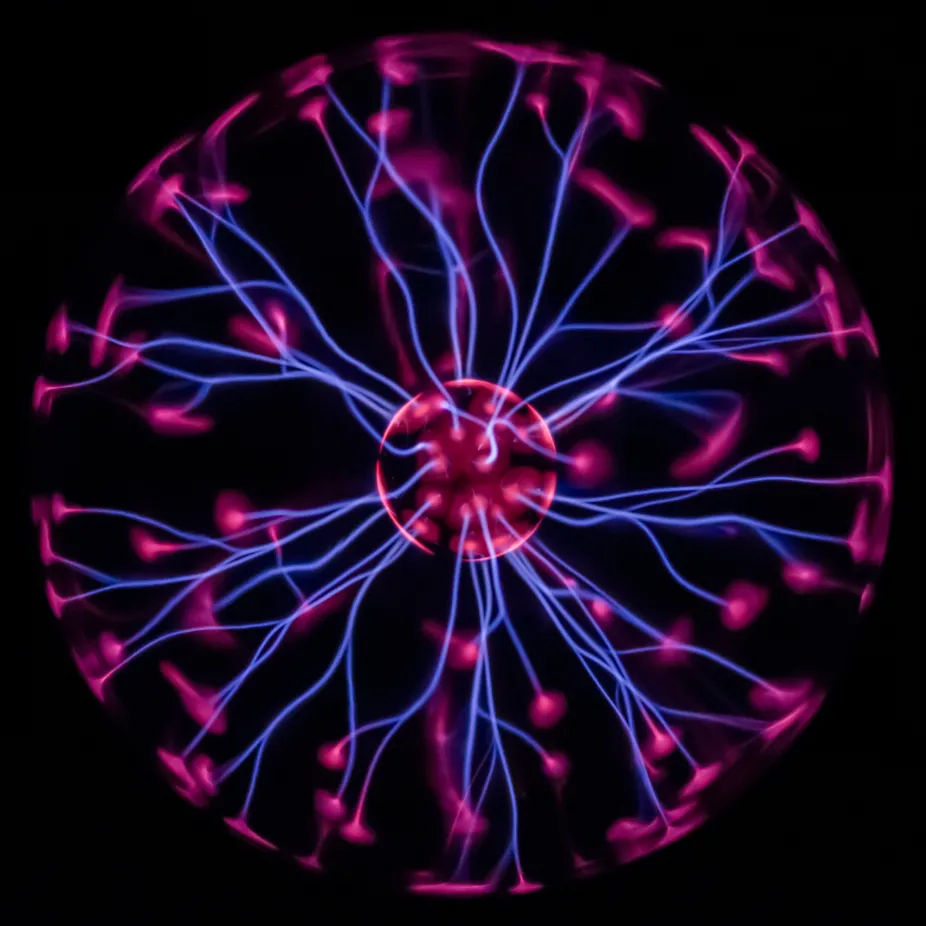

How is Plasma Formed?
Plasma is typically formed when a gas is heated to extremely high temperatures, usually thousands of degrees. At these extreme temperatures, gas particles gain so much energy that electrons are stripped away from atoms in a process called ionization. This creates the free electrons and ions that characterize plasma. Stars and the sun are the most common natural examples of plasma, where the intense heat causes hydrogen and helium atoms to ionize and radiate energy.
Plasma can also be created artificially through electrical discharge or electromagnetic fields. Lightning is a natural example of plasma creation, where the intense electrical energy ionizes air molecules. Plasma has unique properties: it conducts electricity, responds strongly to magnetic and electric fields, and can reach temperatures exceeding millions of degrees in stellar interiors. Plasma is used in modern technology including fluorescent lights, plasma televisions, and fusion reactors for experimental energy generation.
For references of the different states of matter, take a look at our other pages.
